Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mukai, Hiroki*; Hirose, Atsushi*; Motai, Satoko*; Kikuchi, Ryosuke*; Tanoi, Keitaro*; Nakanishi, Tomoko*; Yaita, Tsuyoshi; Kogure, Toshihiro*
Scientific Reports (Internet), 6, p.21543_1 - 21543_7, 2016/02
Times Cited Count:136 Percentile:96.59(Multidisciplinary Sciences)Yaita, Tsuyoshi
Hoshasen To Sangyo, (138), p.13 - 16, 2015/04
no abstracts in English
Yaita, Tsuyoshi
Petorotekku, 37(5), p.329 - 333, 2014/05
no abstracts in English
 TiO
TiO under varied surface condition
under varied surface conditionOlivares, R.*; Oda, Takuji*; Oya, Yasuhisa*; Tanaka, Satoru*; Tsuchiya, Kunihiko
Fusion Engineering and Design, 75-79, p.765 - 768, 2005/11
Times Cited Count:9 Percentile:53.1(Nuclear Science & Technology)no abstracts in English
Takahashi, Manabu*; Tanaka, Kazuya*; Tamada, Masao; Aoi, Toru*
Kankyo Kogaku Kenkyu Rombunshu, Vol.41, p.229 - 235, 2004/11
Fibrous metal adsorbent having iminodiacetic acid was synthesized by radiation-induced grafting glycidyl methacrylate on nonwoven fabric and subsequent chemical treatment. The degree of grafting calculated by increasing weight after grafting reached 170 % for reaction time of 2h at 40  C. The adsorption characteristics of ferric and manganese ions were evaluated by using the resulting adsorbent with 2.1 mmol/g-adsorbent function group of iminodiacetic acid. Each distribution coefficient of ferric and manganese ion deceased with increase of another coexist ion. Both ferric and manganese ions were completely removed by the adsorbent column at the space velocity of 1000h
C. The adsorption characteristics of ferric and manganese ions were evaluated by using the resulting adsorbent with 2.1 mmol/g-adsorbent function group of iminodiacetic acid. Each distribution coefficient of ferric and manganese ion deceased with increase of another coexist ion. Both ferric and manganese ions were completely removed by the adsorbent column at the space velocity of 1000h . Adsorption capacities of both ions were reduced to 80% after 5 times reputation of adsorption and desorption.
. Adsorption capacities of both ions were reduced to 80% after 5 times reputation of adsorption and desorption.
 TiO
TiO
Ryan, O.*; Oda, Takuji*; Oya, Yasuhisa*; Tanaka, Satoru*; Tsuchiya, Kunihiko
JAERI-Conf 2004-012, p.136 - 139, 2004/07
no abstracts in English
Kobayashi, Kazuhiro; Hayashi, Takumi; Iwai, Yasunori; Asanuma, Noriko; Nishi, Masataka
Fusion Science and Technology, 41(3), p.673 - 677, 2002/05
no abstracts in English
Oda, Chie; Ikeda, Takao*; Shibata, Masahiro
JNC TN8400 99-073, 112 Pages, 1999/11
In the safety assessment for geological disposal of high-level radioactive wastes (HLW), distribution coefficients (Kd) and diffusion coefficients of radionuclides are used to estimate the migration of radionuclides in a near-field of repository.  Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
 10
10 [ml/g], 10
[ml/g], 10
 10
10 [ml/g] and 10
[ml/g] and 10
 10
10 [ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10
[ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10 [m
[m /sec] and l0
/sec] and l0 [m
[m /sec] for dry densities of 0.4[g/cm
/sec] for dry densities of 0.4[g/cm ] and 1.0[g/cm
] and 1.0[g/cm ], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO
], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
 ZrO
ZrO packed bed
packed bedKawamura, Yoshinori; Enoeda, Mikio; Okuno, Kenji
Fusion Engineering and Design, 39-40, p.713 - 721, 1998/00
Times Cited Count:8 Percentile:57.22(Nuclear Science & Technology)no abstracts in English
Sakamoto, Yoshiaki; Onuki, Toshihiko;
Radiochimica Acta, 66-67, p.285 - 289, 1994/00
no abstracts in English
Takebe, Shinichi; Mukai, Masayuki; Komiya, Tomokazu; Kamiyama, Hideo
JAERI-M 93-034, 15 Pages, 1993/02
no abstracts in English
 Co,
Co,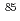 Sr and
Sr and  Cs in a porous tuff; Mechanisms and kinetics
Cs in a porous tuff; Mechanisms and kineticsC.K.Park*; S.I.Woo*; Tanaka, Tadao; Kamiyama, Hideo
Journal of Nuclear Science and Technology, 29(12), p.1184 - 1193, 1992/12
no abstracts in English
; Kubota, Masumitsu
Journal of Nuclear Science and Technology, 27(8), p.743 - 749, 1990/08
no abstracts in English
Proc.9th Symp.on ISIAT 85, p.551 - 552, 1985/00
no abstracts in English
JAERI-M 83-235, 171 Pages, 1984/02
no abstracts in English
JAERI-M 5878, 124 Pages, 1974/10
no abstracts in English
Terashima, Motoki; Tachi, Yukio; Fujiwara, Kenso; Iijima, Kazuki; Shimoda, Satoko*; Akagi, Yosuke*; Kato, Hiroyasu*
no journal, ,
no abstracts in English